One of the ways in which this pH change can be effectively controlled is by the addition of a specie which balances out this charge and offers reactivity against either OH(-) or H3O(+) species. Such addition of species to a solution in order to offer a chemical equilibrium protection against pH changes is called “buffering”. In hydroponics, solutions are most often “buffered” using ammonia, however, we can carry out simulations to see how this two ions act against “acid” or “base” additions and see how the entire hydroponic system reacts to this.
The simulations are carried out using the Maxima software and all the equation systems are generated according to equilibrium equations, mass balance equations and charge balance equations (this is called systematic study of the chemical equilibrium). Concentrations for all the buffering agents were treated as 0.1 mM.
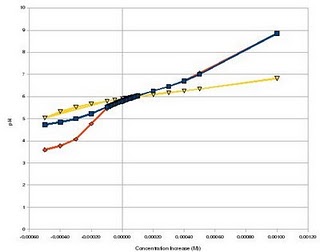
Long story short, the graph below shows the results for my simulations of “acid” or “base” additions using three different buffer agents. The blue line shows the change when citric acid/citrate is used as a buffering agent, the orange line shows when ammonia is used as a buffering agent and the yellow line shows when carbonate/citric acid is used as a buffering agent.
– –
–
As it can be seen, citrate provides very good buffering capacity towards acid pH values while it’s buffering potential towards basic pH values becomes lesser. Ammonia provides almost no buffering potential towards acid pH values while it provides almost the same buffering effect towards basic pH values than citric acid. Note that basic pH buffering seems the same for both buffers because here the effect of the phosphate ions inside the solution becomes more prominent.
–
However, when carbonate/citrtic acid is used as a buffering agent, it suddenly turns the solution’s buffering power towards both acid and basic pH values, extremely high. That said, it should be expected for a solution with this buffering mix to last several times more than a regular solution without needing any pH adjustments. In practice, the preparation of this solution has given me at least three weeks of plant intake without any need for pH adjustment.




