Truly Cleaning Your Hydroponic System : The Fenton Process and Chemistry
In the late 19th century, Henry John Horstman Fenton discovered a chemical process which was able to oxidize the most resilient organic molecules and turn them into harmless chemicals. As a matter of fact, Fenton’s process was so revolutionary that it sprouted a whole new area of research called Fenton chemistry in honor of its discoverer. What was this wonderful discovery ? Within the next few paragraphs you will learn what the Fenton reagent and process are all about and – most importantly – how this process can help you clean, I mean REALLY clean, your hydroponic system between growth cycles.
–
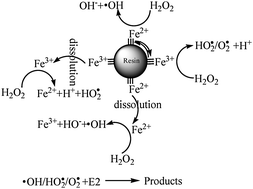
If you have been preparing your own hydroponic solutions and you have been using hydrogen peroxide then you already have most of the things you need to do some Fenton chemistry. The process basically works by adding a source of iron ions (they must NOT be chelated, like iron (II) sulfate) and hydrogen peroxide to a water solution. The iron ions then catalyze a series of reactions that generate powerful oxidizing radicals that destroy almost all harmful organic substances within your system. The iron ions are KEY to the Fenton process since they allow peroxide to generate this extremely reactive substances that are never present when peroxide exists on its own (reactions shown above). Research has found – for example – that phenol (a common chemical used as a model contaminant) remains unchanged in the presence of large concentrations of hydrogen peroxide while it is quickly destroyed in the presence of the Fenton reagent (Iron ions plus hydrogen peroxide). So what do you need to do ?
- First of all you should add about 0.2g of Iron (II) sulfate per liter of solution you will be using to clean your system.
- Then you should set your pH to a level between 3 and 3.5 using a STRONG non-organic acid such as nitric or sulfuric acid. You should NOT use citric, acetic or phosphoric acids since they lower the effectiveness of the Fenton reaction.
- Add as much peroxide as you would add to regularly clean your system. About 70mL of 50% hydrogen peroxide for each liter of solution works very well.
- Circulate the Fenton cleaning solution for at least 6 hours.
- Wash your system with water until no Fenton solution remains.