Methods to Enhance Terpene Production
Introduction
Terpenes are a large and diverse group of natural compounds that constitute the most abundant class of plant secondary metabolites. These volatile organic compounds play critical roles in plant defense against herbivores and pathogens, mediate plant-plant communication, and attract pollinators (1). Beyond their ecological functions, terpenoids have immense commercial value in pharmaceutical, food, cosmetic, and agricultural industries (2). The increasing demand for these compounds in essential oil crops such as mint, citrus, lavender, and other aromatic plants has driven research into methods for enhancing their production. This article reviews scientifically validated approaches to boost terpene biosynthesis in commercially relevant crops.

Understanding Terpene Biosynthesis
Before discussing enhancement methods, it is important to understand the fundamental pathways of terpene production. Plants synthesize terpenoids through two independent but interconnected pathways: the cytosolic mevalonate (MVA) pathway and the plastidial methylerythritol phosphate (MEP) pathway (3). The MVA pathway primarily produces sesquiterpenes and triterpenes, while the MEP pathway generates monoterpenes and diterpenes. Both pathways produce the universal precursors isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DMAPP), which serve as building blocks for all terpene structures.
Terpene synthases (TPSs) are the key enzymes responsible for converting these precursors into the diverse array of terpene structures found in nature (3). In aromatic plants like mint and citrus, these compounds are produced and stored in specialized structures called glandular trichomes and oil glands, respectively. The expression of TPS genes and the activity of these enzymes are tightly regulated by environmental factors and developmental signals, making them prime targets for enhancement strategies.
Controlled Drought Stress Management
Controlled water deficit represents a powerful tool for enhancing terpene production in many aromatic crops. Plants respond to drought by upregulating the biosynthesis of protective secondary metabolites, including terpenoids (4). This response helps plants cope with oxidative stress and signals other plant tissues to activate defensive mechanisms.
Research on medicinal plants has shown that moderate drought stress significantly increases terpenoid content. A study on Bupleurum chinense demonstrated that drought stress stimulated the terpenoid backbone and triterpenoid biosynthesis pathways, leading to increased saikosaponin accumulation (5). Similarly, work on cumin plants revealed that drought-stressed plants showed significant increases in terpene levels alongside upregulation of key biosynthetic genes including 3-hydroxy-3-methylglutaryl-CoA reductase (HMGR) and geranyl diphosphate synthase (GPPS) (6).
In basil and other members of the Lamiaceae family, moderate drought conditions enhanced sesquiterpene production while also improving the overall quality of essential oils (7). The optimal level of drought stress varies by species and growing conditions. Excessive water deficit can inhibit photosynthesis and reduce overall biomass, ultimately decreasing total terpene yield despite higher concentrations per unit mass. Growers should aim for moderate stress that maintains plant health while triggering enhanced secondary metabolism.
Temperature Optimization
Temperature plays a dual role in terpene biosynthesis, affecting both enzyme activity and gene expression. Research on birch and aspen demonstrated that elevated night-time temperatures significantly increased daytime terpenoid emissions. Plants grown with night temperatures of 18 to 22 degrees Celsius showed substantially higher emissions of sesquiterpenes and certain monoterpenes compared to those at lower temperatures (8).
Temperature affects terpene production through multiple mechanisms. Higher temperatures increase the volatility of terpenes, potentially leading to greater emissions from storage structures. More importantly, temperature influences the expression of genes encoding enzymes in both the MEP and MVA pathways (2). However, excessively high temperatures can denature enzymes and degrade already-produced terpenes, so careful monitoring is essential.
For citrus crops, temperature during fruit development significantly affects the terpene profile of essential oils extracted from peels (9). For most aromatic crops, maintaining daytime temperatures between 25 and 30 degrees Celsius with slightly lower night temperatures of 18 to 22 degrees Celsius appears optimal for terpene production. This temperature differential mimics natural conditions and supports robust secondary metabolism without inducing heat stress.
| Crop Type | Optimal Day Temperature | Optimal Night Temperature | Effect on Terpenes |
|---|---|---|---|
| Mint species | 25-30°C | 18-22°C | Enhanced monoterpene production |
| Citrus species | 24-28°C | 16-20°C | Improved essential oil quality |
| Basil and herbs | 26-30°C | 18-22°C | Increased sesquiterpene content |
| Lavender | 22-28°C | 15-18°C | Enhanced linalool production |
Nutrient Management Strategies
Soil nutrient availability profoundly impacts terpene biosynthesis through its effects on carbon and nitrogen allocation. The carbon-nutrient balance hypothesis suggests that when nitrogen is limiting, plants allocate more carbon to secondary metabolites like terpenes rather than to nitrogen-rich primary compounds such as proteins (10).
Phosphorus and potassium play particularly important roles in terpene production. Phosphorus is essential for the production of the phosphorylated precursors DMAPP and IPP, while potassium affects enzyme activation and osmotic regulation under stress conditions. Moderate nitrogen limitation during the reproductive phase can enhance terpene production by shifting metabolism toward secondary compound synthesis (10).
In mint cultivation, nutrient management significantly affects essential oil yield and composition. Studies have shown that excessive nitrogen application can reduce menthol content while promoting vegetative growth at the expense of oil production (11). Sulfur supplementation deserves special attention as this element is incorporated into certain terpenes and affects the overall terpenoid profile. Research has shown that sulfur-containing amendments can enhance the production of sulfur-bearing terpenes while supporting general secondary metabolism.
Elicitor Application
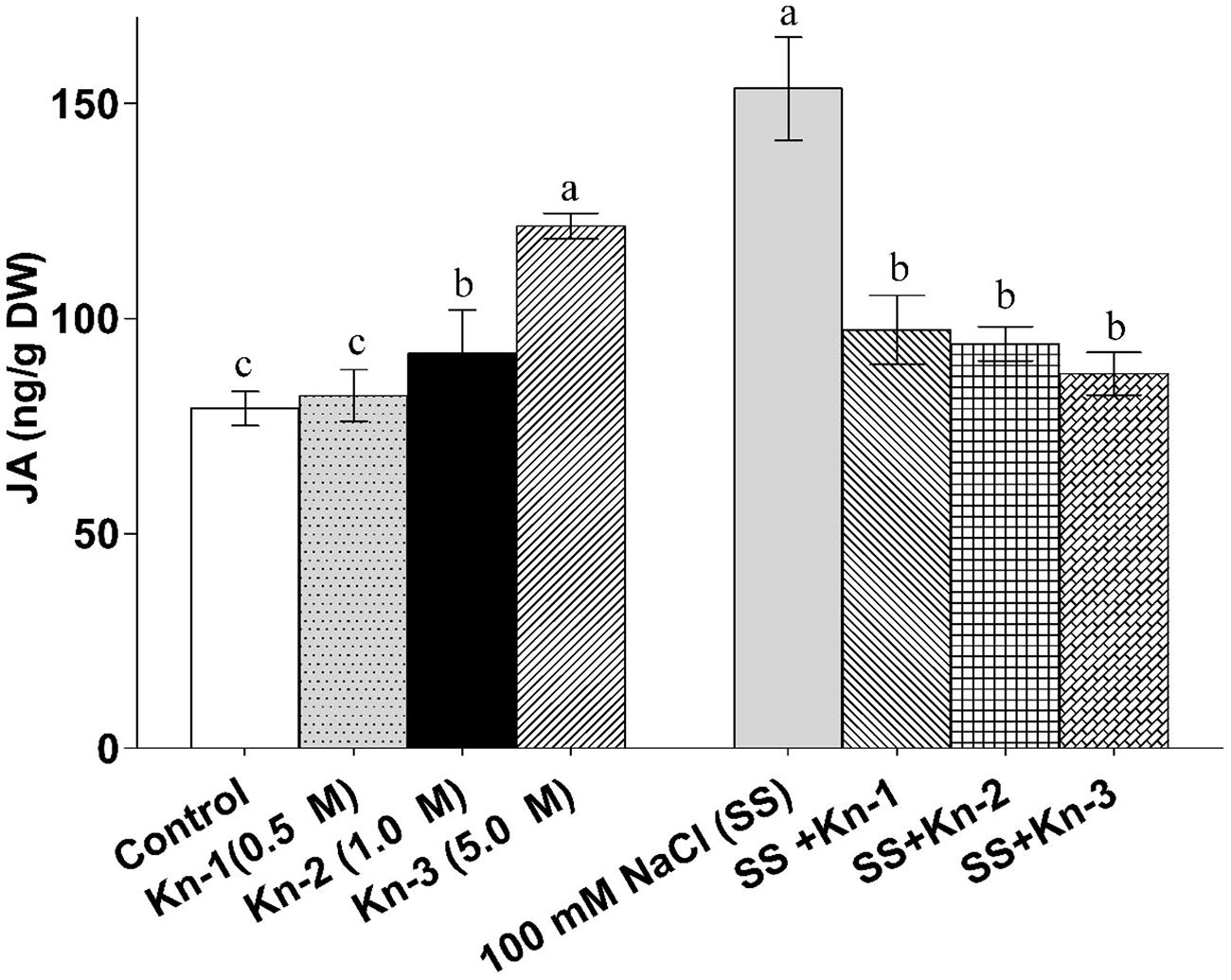
Plant hormones and signaling molecules can act as powerful elicitors of terpene biosynthesis. Methyl jasmonate (MeJA) is the most extensively studied elicitor, with numerous studies demonstrating its ability to dramatically increase terpenoid production (2).
MeJA treatment induces the expression of TPS genes and upregulates the entire terpenoid biosynthetic pathway. In Norway spruce, MeJA application increased terpene emissions by more than 100-fold for linalool and over 30-fold for sesquiterpenes (12). The hormone mimics the plant’s natural defense response to herbivore damage, triggering a cascade of gene expression changes that result in enhanced secondary metabolism.
Salicylic acid represents another important elicitor that can promote terpenoid biosynthesis. Research has shown that salicylic acid upregulates key enzymes in the terpenoid pathway, including farnesyl pyrophosphate synthase (FPPS) in various species (12). In mint species, jasmonate application has been shown to enhance both the quantity and quality of essential oils, particularly increasing the production of oxygenated monoterpenes like menthol and menthone. The optimal concentration and timing of elicitor application depend on the target species and desired terpene profile.
Transcription Factor Regulation
Understanding the transcriptional regulation of terpene biosynthesis opens possibilities for targeted enhancement. Several families of transcription factors (TFs) play crucial roles in controlling terpenoid production, including WRKY, MYB, AP2 or ERF, bHLH, and NAC families (13).
These transcription factors respond to environmental signals and developmental cues by binding to specific promoter regions of genes involved in terpene biosynthesis. For example, WRKY transcription factors regulate sesquiterpene artemisinin synthesis in Artemisia annua and diterpene biosynthesis in rice (13). In citrus, the transcription factor MYC5 has been identified as crucial for oil gland development and the biosynthesis of essential oils (14). While direct genetic manipulation of transcription factors requires advanced techniques, understanding their role helps in timing environmental interventions to coincide with periods of high TF activity.
Metabolic Engineering in Mint Production
Mint species, particularly peppermint and spearmint, represent important commercial sources of monoterpene essential oils. The monoterpenoid biosynthesis pathway in mint is well characterized, making these crops attractive targets for metabolic engineering approaches to enhance oil production (15).
Research has demonstrated that overexpressing genes encoding enzymes in the MEP pathway, particularly 1-deoxy-D-xylulose 5-phosphate reductoisomerase (DXR), can increase essential oil yields in peppermint. The most encouraging results were obtained when multiple genes were manipulated simultaneously. Plants where DXR was overexpressed and menthofuran synthase was down-regulated showed oil yield increases of up to 61% over wild-type controls while reducing undesirable by-products (16).
Another successful strategy involved overexpression of lipid transfer proteins, which increased trichome size and enhanced monoterpenoid production. Plants expressing tobacco lipid transfer protein showed increases in limonene levels of 1.6-fold and dramatic increases in other monoterpenes (15). While metabolic engineering requires sophisticated molecular biology techniques, these advances demonstrate the substantial potential for enhancing terpene production through targeted genetic modifications.
Agronomic Practices for Enhanced Production
Beyond molecular and environmental approaches, specific agronomic practices can significantly impact terpene yields in essential oil crops. For mint cultivation, planting method, timing, and plant density all influence essential oil production (11).
Ridge planting systems have been shown to provide superior results compared to flat-bed cultivation. Studies on menthol mint demonstrated that plants grown on ridges with optimal spacing of 166,666 plants per hectare yielded maximum essential oil content while reducing water requirements and accelerating crop maturity by approximately 30 days (11). The timing of planting also significantly affects oil yield, with early season planting generally producing higher essential oil content and better quality profiles.
For citrus crops, proper handling during harvest and post-harvest processing critically affects terpene retention. Cold-pressing methods preserve more volatile terpenes compared to heat-based extraction, and storage conditions must be carefully controlled to prevent oxidation and degradation of essential oils (17).
Harvest Timing Considerations
The timing of harvest critically affects the final terpene content of plant material. Terpene concentrations fluctuate throughout plant development and can vary substantially even over the course of a single day due to circadian regulation (12).
For many aromatic plants, terpene content peaks during specific developmental stages. In mint, essential oil content typically reaches maximum levels just before full flowering. For citrus, the maturity stage of fruit significantly influences both the quantity and composition of peel oils, with different terpene profiles characterizing immature versus fully mature fruit (9).
Harvesting during the morning hours, after dew has evaporated but before peak temperatures, often captures plants at their maximum terpene content before heat-induced volatilization occurs. Post-harvest handling also significantly impacts terpene retention. Rapid drying at moderate temperatures (below 30 degrees Celsius) and protection from light help preserve volatile terpenes. Proper curing in controlled environments allows for the gradual breakdown of chlorophyll while maintaining terpene content.
| Crop | Optimal Harvest Stage | Time of Day | Post-Harvest Consideration |
|---|---|---|---|
| Peppermint | Just before full bloom | Mid-morning | Rapid drying at 25-30°C |
| Spearmint | Early flowering | Morning hours | Shade drying preferred |
| Citrus peels | Fully mature fruit | Any time | Cold-press immediately |
| Basil | Before flowering | Early morning | Quick drying essential |
Integrated Enhancement Strategies
The most effective approach to enhancing terpene production often involves combining multiple strategies rather than relying on a single method. Environmental factors interact in complex ways, and their effects on terpene biosynthesis can be synergistic (10).
A practical integrated approach for mint cultivation might include selecting cultivars with naturally high terpene production as the foundation, implementing controlled drought stress in the final weeks before harvest, optimizing the nutrient regime to favor secondary metabolism with moderate nitrogen restriction during flowering, applying elicitors such as methyl jasmonate at strategic developmental stages, using appropriate planting methods and densities, and timing harvest to coincide with peak terpene accumulation.
For citrus production, an integrated strategy would focus on temperature management during fruit development, appropriate irrigation scheduling to avoid excessive vegetative growth, balanced fertilization that does not over-supply nitrogen, and optimization of harvest maturity and processing methods to preserve volatile compounds.
Challenges and Future Directions
While significant progress has been made in understanding and manipulating terpene biosynthesis, several challenges remain. The genetic regulation of terpenoid production is extremely complex, involving hundreds of genes that respond to multiple environmental signals (3). Predicting how plants will respond to combined stresses or elicitor treatments remains difficult.
Future research should focus on developing more precise tools for monitoring terpene production in real-time, allowing for adaptive management strategies. Advanced metabolic engineering approaches, including CRISPR-based gene editing of regulatory elements, may eventually allow for the creation of plants with constitutively elevated terpene production without the need for environmental manipulation (18). Understanding the molecular mechanisms controlling oil gland and trichome development will also be crucial for maximizing the sites of terpene biosynthesis and storage (14).
Conclusion
Enhancing terpene production in commercially important plants requires a multifaceted approach based on sound scientific principles. Controlled drought stress, when carefully managed, can significantly increase terpenoid concentrations through activation of stress response pathways. Temperature optimization, particularly elevated night-time temperatures, enhances terpene biosynthesis and emission. Strategic nutrient management, including moderate nitrogen limitation coupled with adequate phosphorus and potassium, shifts plant metabolism toward secondary compound production.
The application of elicitors such as methyl jasmonate provides a powerful tool for rapidly inducing terpene biosynthesis. Understanding the role of transcription factors in regulating these pathways helps in timing interventions for maximum effectiveness. In crops like mint where the biosynthetic pathways are well characterized, metabolic engineering offers promising opportunities for substantial yield improvements. Appropriate agronomic practices, including planting methods, spacing, and timing, significantly influence essential oil production. Finally, optimizing harvest timing and post-harvest handling ensures that enhanced terpene production translates into improved final product quality.
As our understanding of terpene biosynthesis continues to grow, new enhancement strategies will undoubtedly emerge. Growers who stay informed about the latest research and are willing to experiment with different approaches will be best positioned to maximize the terpene content of their crops. However, success requires careful attention to plant health, as excessive stress can be counterproductive. The goal is to find the optimal balance that stimulates terpene production while maintaining overall plant vigor and yield. The commercial value of essential oils continues to drive innovation in this field, promising continued advances in our ability to enhance these valuable compounds in aromatic and medicinal plants.