Five dos and don’ts for automated pH control in hydroponics
The pH is one of the most important variables to control in hydroponic culture as it plays a key role in the availability of different ions and their absorption dynamics. Although most growers control pH manually it is often desirable to implement automatic pH control so that you can ensure that your solution always stays within an optimum range. This is especially true in recirculating systems where correcting the pH of the solution after it goes through the plants’ roots is necessary. Today I want to share with you five dos and don’ts when implementing automated pH control in hydroponic culture.
–
–
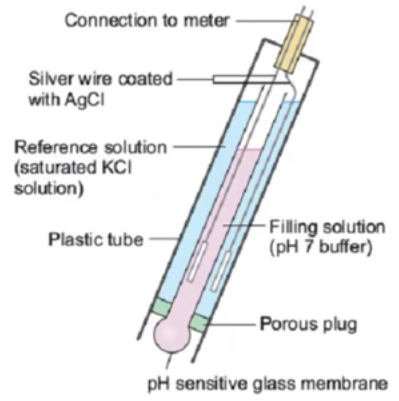
Do test your pH meter frequently with a buffer solution. In hydroponics pH meters can lose calibration rather quickly as a consequence of being immersed in a nutrient solution that is at a lower ionic concentration than what’s ideal for most glass electrodes. This means that testing your pH meter with a buffer solution often – every week is ideal – is necessary in order to ensure that you are getting accurate readings. If the reading is not accurate you can then recalibrate the meter.
Do recondition your pH meter every month. In line with the above and in other to increase the life of a pH meter and each calibration it’s necessary to immerse the pH electrodes in a pH 4 or 7 buffer solution every month – for at least 2-3 hours – to ensure that the ionic content of the electrode is restored and the glass membrane’s responsiveness remains accurate. If you do this your electrode will be happier and you will need to calibrate less frequently. If an electrode is covered in biofilm the putting it in a hot bleach solution for half an hour before the buffer immersion is also necessary.
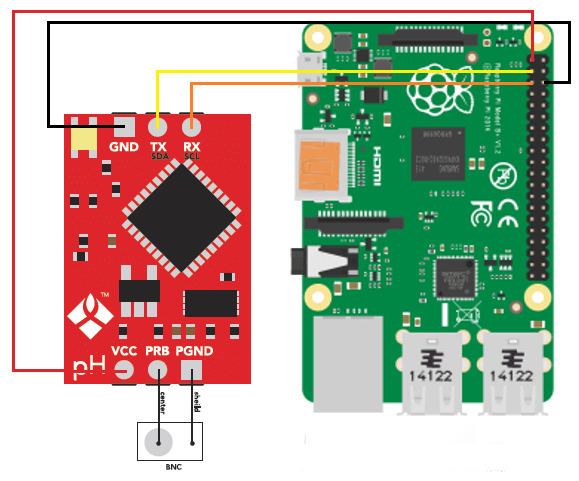
Do use electrodes designed for constant immersion. Regular pH electrodes – including those sold with some automated pH controllers – are not meant to be immersed the whole time and therefore get damaged and lose calibration much more quickly when used in this manner. To get best results use pH electrodes that are fabricated with long term immersion in mind. I wrote a blog post about these electrodes and why they are different than traditional pH electrodes.
Do place your electrode as far as possible from your pH changing inputs. When using a pH controller you should place the pH probe as far away as possible from the place where your pH up/down solutions will enter the hydroponic system. This is so that your pH electrode can get a slow change in pH as the pH up/down is mixed with the entire reservoir. Placing the probe close to the inputs will cause very erratic changes in the pH that do not really reflect the effect of the addition across the entire reservoir.
Do have addition limits in your controllers. Allowing a pH controller to add as much substance as needed to correct the pH can be a very bad thing to do. This is because several things can go wrong – pH probe losing calibration, controller getting damaged, electrical noise etc- that can cause unnecessary levels of addition that can kill an entire crop. Always have controllers where maximum additions per unit of time can be specified so that the possibility of this happening is minimized.
–
–
Don’t rely on a single pH probe. Although single probe controllers are the most common they can also be the most dangerous. A pH probe can get damaged, it can lose calibration or it can give erratic readings due to other reasons (for example electrical interference from other things in the reservoir). Therefore it’s always best to use two-probe controllers where readings are always verified across the two probes to ensure that the reading the controller is getting is accurate. If you have a commercial enterprise then this is a must, you wouldn’t want to lose an entire crop due to a bad pH probe adding a ton of acid/base to your solution.
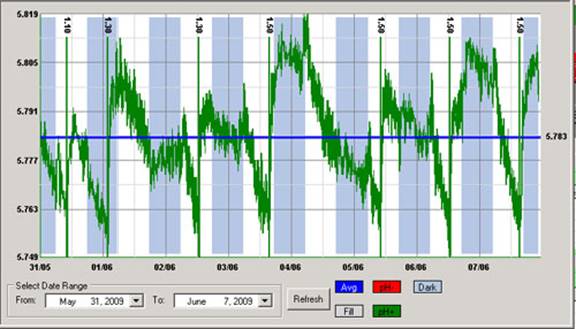
Don’t aim for a specific pH value. A pH controller should not aim for a specific value of pH but to maintain pH within an adequate range. Usually the best way for a controller to act is to have a range with high/low thresholds where the controller will act to take the pH to the middle of the range when these thresholds are exceeded. For example a controller can be told to maintain pH in the 5.6-6.4 region and then it will act whenever the pH reaches 5.6 or 6.4 to take it back to 6 when any one of these two thresholds is breached. However if the pH is at 6.4 and the controller drops it to 5.8 it will not try to then bring the pH up (because it’s above the lower threshold).
Don’t place your pH probes near pumps or other electrical equipment in reservoirs. A pH probe takes an electrical measurement and is therefore prone to electrical interference. Having a pH probe close to other electrical equipment – especially those that draw significant current – can cause those wires to induce currents in the pH probe wires and generate all sorts of issues with pH readings. Always place pH probes away from pumps and ensure the pH probe and pump wires are never tangled together.
Don’t use very concentrated acid/base input solutions. A pH controller will be doing very fine control over a small pH range so it won’t need a very large amount of acid/base to do this job. Using very concentrated acid/base can cause the pH controller to completely overshoot its targets and cause it to either cause the system to get into an undesirable state – for example a very low pH if the controller can only add acid – or enter a loop where acid additions are followed by base additions in an endless cycle. Usually you want your acid/base mixtures to be concentrated enough to shift the pH over their addition volume but not much more than that. Strong acids/bases in the 10-20% concentration range are usually more than enough for this job.
Don’t ignore your controller’s data. A pH controller will do its job – control pH within a range – but it will not tell you whether your system is doing ok or not from a plant-health perspective. How often your pH controller has to add acid/base and how much acid/base it’s using to perform its job are important pieces of information that you need to take into account in order to ensure that your system is working properly. Remember that pH controlling substances often also contribute nutrients – like phosphorous or potassium – so it’s important to keep all these additions in check.
Of course pH control is no simple task and different pH controllers will have different advantages and disadvantages. However doing what you can to ensure proper maintenance – cleaning, conditioning electrodes, having proper placement, etc – can go a long way in ensuring that your setup behaves as ideally as possible. If you can then I would advice you build your own controllers using things like arduinos, raspberry pi computers and robust immersion pH probes so that you can have an optimum setup that can deliver all the advantages of pH control with as few disadvantages as possible. I’ll write about building your own pH controller in a future post.