Advanced phosphorous fertilizers: Are polyphosphates worth it?
If you look into mineral phosphate fertilizers, most of them are of the orthophosphate variety, where phosphorous is present in the form of PO4-3 anions with varying degrees of hydrogen additions depending on the charge balance of the salts. However, there are several different varieties of phosphorous that can be used to fertilize crops. Since the 1970s, polyphosphates have been researched and sold by several different fertilizer companies as a “better way” to fertilize crops. In this post I am going to talk about what polyphosphates are, what the differences with regular orthophosphate fertilizers are, and whether it is worth it or not to replace your current phosphorous fertilization for a regime including or consisting exclusively of these polyphosphates.
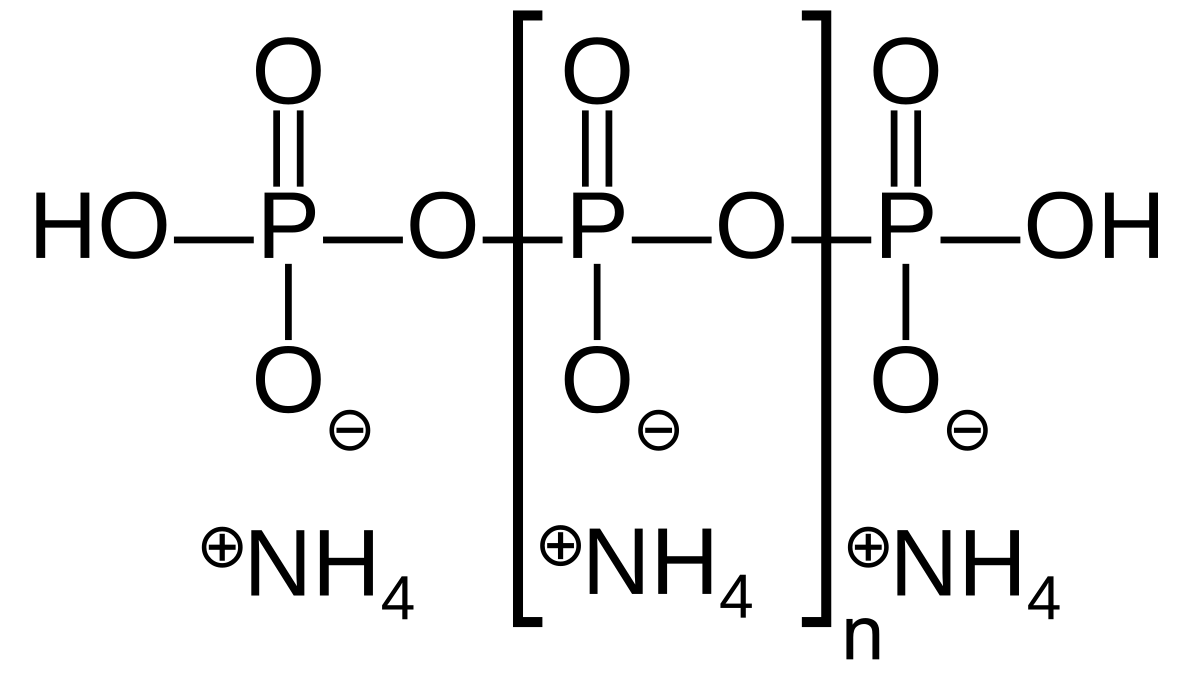
Traditional fertilizers like Mono Potassium Phosphate, MKP (KH2PO4) will contain phosphorous in a chemical state that is readily available to plants. The HPO4-2 and H2PO4– that are generated from this salt in water at a pH between 6-7 are favorably and effectively taken up by plants under normal conditions. However, upon significant presence of calcium/magnesium minerals or high pH levels, it is common for a lot of the phosphorous to become trapped in the form of insoluble phosphates. These calcium and magnesium phosphates will be unavailable to plants and the soil will quickly become P limited, making P fertilization difficult due to the eagerness with which the soil chemistry can sequester the added phosphate.
Polyphosphates like ammonium polyphosphate (APP), where the phosphorous is not present as single phosphate anions but as a complex P polymer, can overcome some of the above problems as their tendency to form insoluble salts with cations is suppressed and their solubility is significantly higher. Their use in calcium-rich soils has been proven experimentally multiple times, the following reference provides an example of this (1). However, is there any benefit provided beyond their superiority in this type of high pH and high Ca conditions?
The chemical properties of APP have been extensively studied and we know that many of their benefits in comparison with orthophosphate (OP) salts are eliminated by a simple move towards acidic pH (2,3). Field experiences have shown that when the soil conditions are not this bad, the differences between APP and OP are expected to be low (4,5). Under normal pH and ion-concentration conditions, APP seems to provide very similar results to normal sources of phosphate, as it will tend to hydrolyze and form these phosphates with time anyway. This effect can be especially dramatic in more acidic media, where the decomposition of these phosphates can be quite rapid (6).

To sum things up, under normal conditions, polyphosphate is no better than your normal sources of phosphorous. If you are running a hydroponic setup within a normal pH range and nutrient concentrations, polyphosphates are just a more expensive way to add phosphorous to your system, they will likely provide no added benefit in terms of yields or crop health compared to using regular phosphate fertilizers. However, if you are growing your crops in a Ca-rich soil that is particularly high pH, where P sequestration due to precipitation is a substantial issue, then polyphosphates offer an alternative method of fertilization that is likely to increase yields against normal orthophosphate fertilizers.



